Abstract
Introduction: The prognosis for acute myeloid leukemia (AML) patients relapsing after allogeneic HCT (allo-HCT) is poor with a 3 year overall survival of <5% for patients relapsing within the first 6 months after allo-HCT. Our group and others have shown that AML relapse after allo-HCT in humans is often associated with the downregulation of genes involved in immune surveillance, including many genes that regulate antigen presentation by Major Histocompatibility Complex (MHC) Class II. However, very few mouse models of AML immune escape after allo-HCT exist and detailed genomic characterization of the relapse/resistant tumors is lacking.
Methods: We utilized a C57Bl/6 (B6) PML-RARA knock-in mouse model of acute promyelocytic leukemia (APL) to examine the genetic changes that contribute to AML relapse after MHC mismatched allo-HCT. Lethally irradiated B6 recipients were reconstituted with BALB/c bone marrow (BM) alone or BM supplemented with B6 APL cells (tumor 18853 or 17857). Cohorts of mice were then left untreated or injected with allogeneic BALB/c T cells (allo-T). After demonstrating in vivo allo-T cell resistance (alloTr), we performed whole exome sequencing (WES) and transcriptome profiling (RNA-seq) on the (i.) "parental" tumor, (ii.) "passage only" control tumors that underwent allo-HCT without allo-T cells, and (iii.) alloTr tumors. The expression of immune modulating and MHC Class I and Class II molecules were examined by flow cytometry.
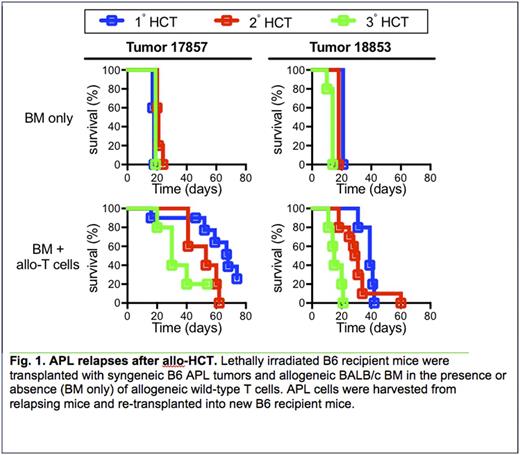
Results: By applying selective therapeutic pressure to tumor cells by means of serial allo-HCT we successfully generated APL tumors that are resistant to allo-T cells (Fig. 1). All mice transplanted without T cells died with a median survival time (MST) between 14 and 20 days. Mice injected with APL tumor 17857 and allo-T cells displayed a significantly decreased MST from 67 days to 30 days during progression from the 1° HCT to the 3° HCT, respectively (P=0.03). Similarly, mice injected with APL tumor 18853 and allo-T cells displayed a significantly decreased MST from 39 days to 15 days during progression from the 1° HCT to the 3° HCT (P=0.004). Of note, nearly all of the relapsed mice treated with allo-T cells presented with hind end paralysis at the time of sacrifice. No paralysis was observed in the passage only controls. Histopathology of the relapsed mice revealed alloTr tumor within the BM that extended into the vertebral canal and musculature surrounding the vertebrae and ribs, and surrounding the spinal and vertebral nerves. Almost no APL cells were detected in the spleen, a primary site of relapse in passage only controls. Overall, 88 and 116 total mutations with variant allele frequencies (VAFs) >5% were detected in at least one of the four samples in the 18853 and 17857 alloTr APL tumors, respectively. Most relevant to our studies, 22 and 19 VAFs were specific to the alloTr 18853 and 17857 tumors, respectively. Restricting the analysis to missense single nucleotide variants (SNVs) with VAFs >5% showed that 9 missense mutations were specific to the 18853 tumors and 4 to the 17857 alloTr tumors. Further, 4 and 5 mutations were specific to the passage control 18853 and 17857 tumors, respectively, suggesting that the subclone(s) containing these mutations were possibly lost upon treatment with allogeneic T cells. Although our WES data suggest selection and/or generation of an APL subclone upon application of allogeneic T cell pressure, no mutations were observed in MHC genes or in genes involved with immune function. Immunophenotypic analysis of the alloTr cells by flow cytometry revealed a 2-fold decrease in expression of MHC Class I (H2Db) in both alloTr samples. The 18853 alloTr tumor also displayed decreased expression of MHC Class II (I-A) and the co-stimulatory marker OX40L, while the 17857 tumor downregulated the apoptosis mediator CD95 (Fas). There were no differences in expression of H2Kb, PD-L1, E-cadherin, galectin-9, CD40, CD80 and CD86 between the passage only control and alloTr tumors. A transcriptional profiling analysis of these samples by RNA-seq is currently ongoing and may provide further mechanistic insights into the genetic or epigenetic events leading to tumor immune escape.
Summary: Using a serial allo-HCT approach, we have successfully generated murine APL cells that are resistant to allogeneic T cells. Our data suggest that loss of MHC class I and other epigenetic changes contribute to relapse in this murine APL model.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.